Bacteria Defense Against Phage Nature Review Microbiology Crispr Rm Abi
Introduction
Prokaryotes evolve genetic material under the constant influence of, or exposure to, mobile genetic elements (MGE) such as plasmids and bacteriophages. Bacteriophages are the most arable biological entities on globe (Bergh et al., 1989; Wommack and Colwell, 2000). Proliferation of bacteriophages occurs in a series of events: attachment or adsorption of a virion to the host cell wall, injection of viral genome (DNA or RNA) through the jail cell membrane, expression of viral genes, viral genome replication in host prison cell, and, as a final stage, progeny virions are released from the lysed host (Sturino and Klaenhammer, 2004). Another class of MGEs is plasmids. Plasmid Dna resides in host cytoplasm either gratuitous or integrated into the host genome sequence. Plasmids can be transferred from a donor prison cell to recipient cell by transformation, conjugation, and transduction (Llosa et al., 2002; Thomas and Nielsen, 2005). Integrated MGEs account for up to 30% of some bacterial genomes (Casjens, 2003; Busby et al., 2013).
Bacteria and their associated bacteriophages undergo continuous cycles of development to generate resistance to each other in both natural and human-made ecosystems (Stern and Sorek, 2011). The bacterial immune mechanisms/systems developed in response to invaders are enormously diverse, and there are potentially many remaining to be discovered. Currently described innate allowed mechanisms involve aversion of phage adsorption or blockage of phage Deoxyribonucleic acid entry. When these mechanisms fail, abortive infection (Abi) comes into activity and triggers the suicide of infected bacterial cells, resulting in the prevention of phage replication, which also benefits the bacterial population adjacent to the infected cell.
A clustered, regularly interspaced, short palindromic repeat (CRISPR) locus is the only known adaptive immune organisation in prokaryotes. A brusque DNA sequence of the phage is integrated into the CRISPR loci and produces sequence-specific immunity against the invading bacteriophage (Hyman and Abedon, 2010; Labrie et al., 2010). Here, nosotros focus on bacterial innate and adaptive immune systems and phage counter-strategies against the CRISPR-cas organisation.
Averting Phage Adsorption/Receptor Mutation
In natural environments, bacteriophages face a vast diverseness of organisms, but generally their host range is limited to a single bacterial species (Hyman and Abedon, 2010). To inject DNA into the target cell, bacteriophages must demark to a specific surface receptor of that cell (Hyman and Abedon, 2010; Labrie et al., 2010). Proteins, polysaccharides, or lipopolysaccharides (LPS) nowadays on the cell surface serve as receptors for bacteriophages. Strategies to avoid phage attachment include modification of the structure of the receptor via mutation and concealing the receptor with a physical barrier (Labrie et al., 2010; Dy et al., 2014). For example, Staphylococcus aureus produces cell wall associated virulence factor (immunoglobulin G-binding poly peptide A) that binds with the Fc fragment of immunoglobulin One thousand (Foster, 2005). Phage adsorption has been shown to improve when bacteria secrete less protein A (Nordström and Forsgren, 1974). Availability of the receptor tin can exist decreased via variation in the phase during which the expression of the receptor is subjected to heritable, reversible switching that ensures the survival of bacteria population heterogeneity. 2 component regulatory organization (BvgAS) was identified in Bordetella by transposon mutagenesis screening (Weiss et al., 1983). Bordetella bronchiseptica shows variation between the Bvg+ phase, required for pulmonary colonization, and the Bvg- phase. Bacteria tin express diverse virulence and colonization factors, forth with the adhesion molecule pertactin, during the Bvg+ phase. Temperate phages showing clan with B. bronchiseptica clinical isolates have been identified that demonstrate the ability to use pertactin as a receptor (Liu et al., 2002).
In many cases, upon infection, lytic phages hydrolyze host Deoxyribonucleic acid, suggesting that the potential for horizontal Dna commutation between an infecting phage and a prophage is significantly lower than in temperate phages (Hendrix, 2003). However, temperate phages have the ability to enter the host prison cell as a prophage dormant state and replicate along with the bacterial genome. Upon interaction of these phages with the host, they not merely change the bacterial phenotype, affecting its virulence, merely as well import new genes into the host (De Paepe et al., 2014).
Vibrio cholerae is host to various bacteriophages (vibrio phages) including temperate phages (kappa-blazon phages) and virulent phages (Mukherjee's cholera phages). These phages were ordinarily used for V. cholerae O1 phage typing (Basu and Mukerjee, 1968). Later, virulent phages were used for the phage typing of V. cholerae O1 (Chattopadhyay et al., 1993) and O139 (Chakrabarti et al., 2000). Filamentous phages, which normally practise non kill the host, also played a significant role in the evolution of V. cholerae biology. These phages human action in horizontal gene transfer (HGT) among V. cholerae strains (Faruque and Mekalanos, 2003). For effective colonization of the abdominal tract, 5. cholerae O1 sero-grouping strains depend on LPS O1 antigen expression. Clinical samples of Five. cholerae possess virulent phages, rather than temperate phages, and their relationship relies on the expression of O1 antigen in wild type strains. When this antigen is under the influence of phase variations, information technology averts phage infection (Seed et al., 2012).
Haemophilus influenza, a gram negative pathogen, possesses numerous genes that can produce phase variation via slipped strand mispairing (Hood et al., 1996; Bayliss et al., 2004). Genetic mutations oftentimes upshot in a change of echo number within, or at, promoter sequences. Inside the gene lic2A, Dna polymerase slippage of tetra-nucleotide repeats results in the synthesis of surface lipo-oligosaccharides (LOS), which can be involved in changing the receptor composition of the HP1c1 phage to shift betwixt sensitive and resistant phenotypes (Zaleski et al., 2005).
Expression of receptors present on the cell surface tin be modified by competing phages. Pseudomonas aeruginosa possesses type IV pilus (TFP) equally its mechanism of pathogenesis and biofilm formation, which tin can be modified by lysogenic conversion. A protein designated Tip, encoded past phage D3112 tin can bind with ATPase of TFP and avert its localization, leading to the loss of surface piliation and also conferring protection from other phages relying on TFP for infective action (Chung et al., 2014).
Phage receptors may be veiled by a capsule. For instance, the K1 sheathing of Escherichia coli interferes directly with phage T7 and prevents its zipper to LPS receptors (Scholl et al., 2005). Along with hiding receptors, leaner may produce decoys to circumvent phage attachment. In the presence of an outer membrane vesicle (OMV), the level of phage T4 can exist reduced, suggesting that OMV shedding into the environs might act equally bait to avert phage attachment. If there is no shedding of OMVs, presence of phage T4 will outcome in an active infection (Manning and Kuehn, 2011).
Host phase variation can have pregnant impact on phage adaptation. Due east. coli phage Mu possesses Gin recombinase that plays a part in the inversion of the 1000 segment, leading to the expression of tail fiber genes that act in determining the phage host range. Similar systems have been identified in the P1 phage and in other phages (Sandmeier et al., 1992). In some phages having tail cobweb operons, shufflons are observed that effect in multiple inversions forth with production of tail fiber types having diverse host specificities (Sandmeler, 1994).
Blockage of Invader DNA
Later the zipper of the bacteriophage to a specific receptor on the surface of a bacterial cell, information technology injects its DNA into the host cell where it utilizes host mechanisms for replication (Marks and Sharp, 2000). Superinfection exclusion (SIE) systems of bacteria human action in blocking invader DNA entry. These systems consist of membrane-associated proteins encoded by phages and protect the lysogenized host from infection past other closely related phages. Streptococcus thermophilus contains TP-J34 phages that demonstrate power to produce the membrane-localized lipoprotein LtpTP-J34, which interacts with the record measure protein of other phages (Bebeacua et al., 2013). In Siphoviridae, since tape measure poly peptide facilitates DNA passage past channel germination, LtpTP-J34 non only blocks the injection process only besides reduces incursion of non-infectious phages. A predicted transmembrane protein, gp15, is produced by E. coli phage HK97 that inhibits the Dna entry of HK97 along with closely associated phage HK75 (Cumby et al., 2012). Although many DNA blocking SIE systems have been recognized, there is a need to report their mechanisms of activeness. In dissimilarity to receptor blocking strategies, SIE systems provide an reward to the bacterium in protecting specific cells and adjacent populations against phage superinfection. After Deoxyribonucleic acid injection, the infecting phage facilitates the DNA ejection of not-infectious phages.
Brake–Modification Systems
If a bacteriophage successfully adsorbs and injects its Dna into the bacterium, various innate defense systems are in place to avoid phage replication. Restriction modification (R-M) systems have the ability to cleave invader DNA. Characteristically, R-G systems consist of restriction endonuclease (REase) and a cognate, methyltransferase (MTase) (Tock and Dryden, 2005). The MTase normally does not modify invader DNA, but acts in self Deoxyribonucleic acid methylation at particular recognition sites. In contrast, REase tin can recognize invader Dna and degrade information technology into harmless fragments. Restriction–modification systems are divided into four types on the basis of their subunit composition, recognition site, and mechanism of action (Roberts et al., 2003). Phages can resist R-M systems by incorporating the modified bases (Samson et al., 2013). Notwithstanding, some bacteria (e.g., McrBC in Eastward. coli) have modification-dependent REases that act only on modified DNA (Stewart et al., 2000). The restriction system (McrBC) was starting time described in E. coli, in 1952 (Luria and Human, 1952). At that time most phage enquiry involved the T-fifty-fifty phages, and McrBC is active against T-even phage variants having not-glycosylated hydroxymethylcytosine (hmC) in their Dna (Revel, 1983). The restriction enzymes are methylation-dependent and cleave the DNA afterward recognition who does not have the strain-specific modification (Noyer-Weidner et al., 1986).
Phages have evolved diverse anti-brake strategies against R-M systems, including the absence of endonuclease recognition sites in their genomes as the result of point mutations. The polyvalent Staphylococcus phage K has no Sau3A sites in its dsDNA genome (Krüger et al., 1987; Tock and Dryden, 2005). Restriction–modification system antiviral efficacy is straight related to the number of recognition sites nowadays in a viral dsDNA genome (Wilson and Murray, 1991). Some phages have overcome R-M systems by acquiring the cognate methylase gene in their genomes (McGrath et al., 1999).
Abortive Infection
A bacterium survives viral assault in the mentioned innate defense systems, but this is non the case in Abi systems. Under the issue of Abi systems, predation by a bacteriophage results in expiry of the bacterium, thus protecting adjacent bacterial populations. Abi systems are often encoded by MGEs such as plasmids and prophages (Samson et al., 2013). These systems have the ability to human activity on any phase of bacteriophage development thus preventing its proliferation. In phage lambda, the RexAB organisation protects lysogenized cells from infection by several other coliphages through inducing loss of membrane potential, resulting in a decreased level of ATP (Snyder, 1995). More than 20 Abis, designated AbiA to AbiZ, take been identified in Lactococcus lactis, a bacterium that encounters bacteriophage attack during its extensive employ in the fermentation process of cheese making (Chopin et al., 2005). At an early phase in the bacteriophage replication cycle, AbiP plays a role in the disruption of both phage DNA replication and temporal transcription (Domingues et al., 2004). Premature lysis of the infected jail cell is induced by AbiZ, ensuring incomplete viral assembly forth with prevention of release of infectious virions (Durmaz and Klaenhammer, 2007). It has been recently shown that Abi systems are mediated by toxin-antitoxin (TA) systems (Fineran et al., 2009). For example, the AbiE system can preclude phage proliferation by inducing bacteriostasis (Dy et al., 2014).
Interference During Assembly
Double strand DNA phages typically possess holin-lysin systems that disrupt their growth cycle, which results in viral progeny release through host cell lysis. Endolysins generally practise not have intrinsic secretory signals, holins accumulate and form lesions in the cytoplasmic membrane thereby regulating the endolysins (phage encoded) to the peptidoglycan and, at a precise time betoken, triggering host prison cell lysis (Shi et al., 2012). Gram positive bacteria contain phage-inducible chromosomal islands (PICI) that deed as phage parasites displaying the power to interfere with phage reproduction (Ram et al., 2012). Among the growing identified family unit of PICIs is the well-studied S. aureus pathogenicity island (SaPI) that carries and spreads virulence factors (Novick et al., 2010). Nether normal weather condition, SaPIs reside in the bacterial chromosome but, upon infection, with exposure of helper phages, become agile and excise, replicate, and package themselves. Currently described SaPIs take the power to bear upon helper phage particle assembly as well as packaging of Deoxyribonucleic acid; however, in dissimilarity to other phage-resistant mechanisms, SaPI activity depends on progression of intracellular phage development to produce a generation of mature phage particles loaded with SaPI Deoxyribonucleic acid instead of phage DNA (Ram et al., 2014). During Abi defense force, the jail cell dies a event of phage infection, phage reproduction is express, and SaPIs spread to adjacent cells. SaPIs utilise various unique strategies to interfere with phage reproduction. They can modify phage capsid protein into small capsids containing the SaPI genome rather than the larger helper phage genome (Ruzin et al., 2001; Ram et al., 2012). Phage packaging interference (Ppi) proteins are encoded by SaPIs that are thought to play a role in blocking the phage terminase minor subunit necessary for phage DNA recognition as well as for initiation of packaging, assuasive the SaPI terminase small subunit to collaborate with the phage-encoded large subunit to facilitate SaPI Deoxyribonucleic acid cleavage for packaging (Ram et al., 2012). A farther interference mechanism involves interruption in late gene activation of phages, which is necessary for phage packaging every bit well equally cell lysis (Ram et al., 2014). V. cholerae comprise a PICI-like chemical element, which acts in the inhibition of virulent phages (Seed et al., 2013), although the mechanism of this activity is yet to exist discovered.
Crispr: Bacteria Adaptive Allowed System
Bacteria CRISPR-cas systems have the ability to target and destroy DNA of MGEs such equally plasmids, phages, transposons, and pathogenicity islands. CRISPR loci and their associated cas genes participate in the adaptive defence force mechanism to protect bacteria against invaders (Deveau et al., 2010; Marraffini and Sontheimer, 2010). A CRISPR locus consists of short arrays of repetitive sequences that are separated by plasmid- or bacteriophage-derived spacer sequences. The mechanism of the CRISPR system against invaders DNA consists of 3 steps: Adaptation (Garneau et al., 2010; Marraffini and Sontheimer, 2010) involves the acquisition of a spacer after recognition and discrimination of two repeat units present inside the CRISPR array. As the spacers are integrated at the leader cease of the CRISPR loci, the position of the spacer in the locus provides a inkling to its conquering result (Deveau et al., 2008). Cas1 and cas2 proteins are essential to accommodation, as they are critical to the acquisition of the spacer.
In the CRISPR expression step, pre-CRISPR RNA (pre-crRNA) transcription occurs by RNA polymerase within the CRISPR assortment. After transcription, the pre-crRNA is cleaved past specific endoribonucleases into small CRISPR RNAs (crRNA). Due to its function, crRNA is also called guide RNA (Brouns et al., 2008; Carte et al., 2008).
In the concluding footstep, interference, multi-poly peptide complexes having mature crRNA recognize and form specific base pairs with invader DNA or RNA (Brouns et al., 2008), resulting in cleavage of crRNA-invader nucleic acrid complex (Figure one) (Garneau et al., 2010).
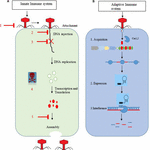
Effigy ane. (A,B) Overview of the innate and adaptive allowed organisation of leaner against bacteriophages. Bacterial anti-phage systems with flat arrow heads. one = averting phage adsorption, ii = blockage of invader DNA entry, 3 = restriction–modification system, 4 = abortive infection, and v = interference during assembly. The correct side represents stages of bacteriophage replication in host cells. (B) Represents the CRISPR-cas allowed system. In the starting time stage, spacer is integrated with the help of Cas1,2 proteins into the CRISPR loci. In second stage, biogenesis and maturation of pre-crRNA into mature crRNA occur. In the final phase, foreign Deoxyribonucleic acid is broken (blue and red stripes) with the help of a specific enzyme.
Counterattack of Invaders Against the Crispr-Cas System
Phages of P. aeruginosa have been institute to encode proteins that testify power to inactivate the CRISPR-cas system. The type I-F system is inhibited by v anti-CRISPR protein families, while the type I-E system is inhibited by four anti-CRISPR protein families (Bondy-Denomy et al., 2013; Pawluk et al., 2014). The genes responsible for encoding of these proteins take been observed in Mu-like phages. The anti-CRISPR operon was establish to be present in the same position in a diversity of Pseudomonas related phages. However, the anti-CRISPR gene complement that comprise the locus varies amongst phages. For instance, of 24 related phages displaying the anti-CRISPR operon, 15 encode the anti-CRISPR genes of both CRISPR arrangement types I-E and I-F, ane encodes type I-Due east only, and blazon I-F only is encoded by other eight (Pawluk et al., 2014). Collectively, ix distinct arrangements were provided by these phages in blazon I-E and I-F anti-CRISPR genes, revealing that these genes re-assort several times via HGT in a mix-and- match fashion. Due to the few homologous sequences, it is difficult to trace the anti-CRISPR poly peptide evolutionary origin. Nevertheless, their frequent occurrence in Pseudomonas phages suggests that they provide substantial evolutionary advantage.
Office of Anti-Crispr Proteins Exhibited By Various Mechanisms
In response to phage set on, CRISPR organisation type I-F activity is categorized into three stages (Figure two): In the first phase, invader phage Deoxyribonucleic acid is recognized and incorporated into the CRISPR array as spacer past cas1 and cas2 proteins. In the next phase, transcription of pre-crRNA occurs, which is cleaved into mature crRNA, facilitated past Csy4 endoribonuclease that remains attached to the iii′ end of crRNA (Haurwitz et al., 2010). This crRNA-Csy4 circuitous interacts with Csy1, Csy2, and Csy3 proteins, resulting in the formation of a Csy circuitous (Wiedenheft et al., 2011). This complex binds with foreign DNA (complementary to crRNA) by surveying the bacterial cell. Upon binding with strange Deoxyribonucleic acid, Cas3 helicase–nuclease poly peptide is recruited by this circuitous resulting in degradation of target Dna (Westra et al., 2012; Huo et al., 2014).
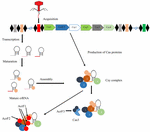
Effigy two. Machinery of action of iii anti-CRISPR proteins. Black diamonds point direct repeats of CRISPR system type I-F separated by spacer sequences (colored boxes). In the presence of cas1,ii proteins, foreign Dna (carmine box) is caused at the CRISPR locus. Subsequently that biogenesis of crRNA, mature crRNA forms in response to pre-crRNA transcription. crRNA-Csy circuitous bounden with Csy1, Csy2, and Csy3, results in the formation of surveillance circuitous. Anti-CRISPR proteins AcrF1 and AcrF2 interact direct with this circuitous and block the binding of DNA. AcrF3 interacts with Cas3 nuclease poly peptide and prevents its recruitment (Maxwell, 2016).
The anti-CRISPR system of type I-F possesses 3 unique proteins, AcrF1, AcrF2, and AcrF3 (Bondy-Denomy et al., 2015). In the Csy complex, the heterodimer of Csy1-Csy2 bind at the five′ end of crRNA, while the monomer of Csy4 binds at the iii′ end, and Csy3 (6 subunits) binds along the RNA spacer (Haurwitz et al., 2010; van Duijn et al., 2012). AcrF1 and AcrF2 proteins interact with the Csy circuitous to block DNA binding with this complex (Figure two). AcrF2 is bound to the heterodimer of Csy1–Csy2 with a stoichiometry of ane anti-CRISPR molecule per complex. This leads to inactivation of the CRISPR-cas system due to blockage of the v′ end. It has been demonstrated that AcrF1 binds along the full length of Csy3. Dissimilar AcrF2, AcrF1 has the ability to bind with the complex even when the complex is already bound to Dna. Hence, both AcrF1 and AcrF2 bind with the Csy circuitous, but with different mechanisms of action. AcrF3 interacts direct with cas3 endoribonuclease to block of its recruitment to the Csy complex (Effigy ii). Accommodation of anti-CRISPR mechanisms to diverse protein sequences indicate independent evolutionary pathways.
Anti-Crispr Genes in MGEs
Survival of MGEs upon invasion into bacteria tin can be increased if they show the ability to inactivate CRISPR-cas systems. Recently, several anti-CRISPR homologs were found inside non-phage-associated genome regions of various Pseudomonas strains. These regions include many genes encoding proteins (probable MGEs) that are agile in Dna transfer and conjugation (Bondy-Denomy et al., 2013; Pawluk et al., 2014). These anti-CRISPR genes tin can increase the survival of MGEs upon inter-strain transfer by inactivating the recipient CRISPR-cas systems.
It is too thought that MGEs possessing anti-CRISPR proteins might play a function in enhancing the virulence of bacterial strains. For example, an agile pathogenetic area containing anti-CRISPR homologs has been identified in clinical isolates of highly virulent P. aeruginosa, and it is believed that this pathogenicity island is transferred via conjugation among P. aeruginosa (Battle et al., 2008). The discovery of anti-CRISPR genes in MGEs suggests that they might play a cardinal office in lateral cistron transfer by permitting invader Dna to evade CRISPR-cas systems.
Summary
Bacteriophages exert strong selective pressure level that plays a significant role in most ecosystems, not but to control the numbers, but also the composition, of the bacterial population. Likewise, bacteria develop immune strategies to avert phage assault by regulating the number and composition of phages, leading to the establishment of predator-prey dynamic equilibrium. Plasmids and prophages that act as a cocky-interested elements play are vital to the development of phage resistance strategies. These elements provide constructive barriers confronting phage infection without compromising the biological integrity of the host cell. Phages continually adapt and evolve in response to bacterial allowed system development. Phages related to Pseudomonas accept adult anti-CRISPR systems to counterattack the CRISPR-cas system blazon I-E and I-F present in the bacterial host.
Future Perspectives
1. There is a need for molecular label of anti-phage systems that are however to be fully understood.
2. The discovery of the Pseudomonas-associated phage anti-CRISPR organisation opens a discussion of whether other bacteria-associated phages take adult similar strategies.
Author Contributions
MS wrote a manuscript while HH, MS, QW, AS, and ZY reviewed and edited manuscript. All authors read and canonical the terminal manuscript.
Conflict of Involvement Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Battle, S. East., Meyer, F., Rello, J., Kung, V. L., and Hauser, A. R. (2008). Hybrid pathogenicity island PAGI-5 contributes to the highly virulent phenotype of a Pseudomonas aeruginosa isolate in mammals. J. Bacteriol. 190, 7130–7140. doi: ten.1128/JB.00785-08
CrossRef Full Text | Google Scholar
Bayliss, C. D., Sweetman, Due west. A., and Moxon, Eastward. R. (2004). Mutations in Haemophilus influenzae mismatch repair genes increase mutation rates of dinucleotide echo tracts only non dinucleotide repeat-driven pilin phase variation rates. J. Bacteriol. 186, 2928–2935. doi: 10.1128/JB.186.ten.2928-2935.2004
CrossRef Full Text | Google Scholar
Bebeacua, C., Fajardo, Fifty., Carlos, J., Blangy, Due south., Spinelli, S., Bollmann, S., et al. (2013). 10-ray structure of a superinfection exclusion lipoprotein from phage TP-J34 and identification of the record mensurate poly peptide as its target. Mol. Microbiol. 89, 152–165. doi: x.1111/mmi.12267
CrossRef Total Text | Google Scholar
Bergh,Ø, Børsheim, K. Y., Bratbak, Thou., and Heldal, One thousand. (1989). High abundance of viruses found in aquatic environments. Nature 340, 467–468. doi: 10.1038/340467a0
CrossRef Full Text | Google Scholar
Bondy-Denomy, J., Garcia, B., Strum, S., Du, M., Rollins, M. F., Hidalgo-Reyes, Y., et al. (2015). Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature 526, 136–139. doi: 10.1038/nature15254
CrossRef Full Text | Google Scholar
Bondy-Denomy, J., Pawluk, A., Maxwell, Thou. L., and Davidson, A. R. (2013). Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493, 429–432. doi: 10.1038/nature11723
CrossRef Full Text | Google Scholar
Brouns, South. J. J., Jore, M. M., Lundgren, M., Westra, E. R., Slijkhuis, R. J. H., Snijders, A. P. L., et al. (2008). Small CRISPR RNAs guide antiviral defence force in prokaryotes. Science 321, 960–964. doi: 10.1126/scientific discipline.1159689
CrossRef Full Text | Google Scholar
Busby, B., Kristensen, D. M., and Koonin, Due east. V. (2013). Contribution of phage-derived genomic islands to the virulence of facultative bacterial pathogens. Environ. Microbiol. 15, 307–312. doi: x.1111/j.1462-2920.2012.02886.x
CrossRef Full Text | Google Scholar
Carte, J., Wang, R., Li, H., Terns, R. M., and Terns, M. P. (2008). Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22, 3489–3496. doi: x.1101/gad.1742908
CrossRef Full Text | Google Scholar
Casjens, S. (2003). Prophages and bacterial genomics: what have we learned and then far? Mol. Microbiol. 49, 277–300. doi: 10.1046/j.1365-2958.2003.03580.x
CrossRef Full Text | Google Scholar
Chakrabarti, A. Chiliad., Ghosh, A. N., Nair, G. B., Niyogi, S. Thou., Bhattacharya, S. Thou., and Sarkar, B. L. (2000). Development and evaluation of a phage typing scheme for Vibrio cholerae O139. J. Clin. Microbiol. 38, 44–49.
Google Scholar
Chattopadhyay, D. J., Sarkar, B. L., Ansari, M. Q., Chakrabarti, B. 1000., Roy, M. K., Ghosh, A. N., et al. (1993). New phage typing scheme for Vibrio cholerae O1 biotype El Tor strains. J. Clin. Microbiol. 31, 1579–1585.
Google Scholar
Chopin, K.-C., Chopin, A., and Bidnenko, East. (2005). Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8, 473–479. doi: 10.1016/j.mib.2005.06.006
CrossRef Full Text | Google Scholar
Chung, I.-Y., Jang, H.-J., Bae, H.-W., and Cho, Y.-H. (2014). A phage protein that inhibits the bacterial ATPase required for blazon IV pilus assembly. Proc. Natl. Acad. Sci. U.S.A. 111, 11503–11508. doi: 10.1073/pnas.1403537111
CrossRef Full Text | Google Scholar
Cumby, N., Edwards, A. M., Davidson, A. R., and Maxwell, Chiliad. Fifty. (2012). The bacteriophage HK97 gp15 moron chemical element encodes a novel superinfection exclusion protein. J. Bacteriol. 194, 5012–5019. doi: 10.1128/JB.00843-12
CrossRef Full Text | Google Scholar
De Paepe, M., Hutinet, Thou., Son, O., Amarir-Bouhram, J., Schbath, Southward., and Petit, M. A. (2014). Temperate phages acquire Dna from defective prophages by relaxed homologous recombination: the office of Rad52-Like recombinases. PLoS Genet. 10:e1004181. doi: ten.1371/journal.pgen.1004181
CrossRef Full Text | Google Scholar
Deveau, H., Barrangou, R., Garneau, J. E., Labonté, J., Fremaux, C., Boyaval, P., et al. (2008). Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400. doi: x.1128/JB.01412-07
CrossRef Total Text | Google Scholar
Deveau, H., Garneau, J. E., and Moineau, S. (2010). CRISPR/Cas system and its role in phage-leaner interactions. Annu. Rev. Microbiol. 64, 475–493. doi: 10.1146/annurev.micro.112408.134123
CrossRef Total Text | Google Scholar
Domingues, Due south., Chopin, A., Ehrlich, South. D., and Chopin, Yard.-C. (2004). The lactococcal abortive phage infection arrangement AbiP prevents both phage DNA replication and temporal transcription switch. J. Bacteriol. 186, 713–721. doi: 10.1128/JB.186.three.713-721.2004
CrossRef Full Text | Google Scholar
Durmaz, E., and Klaenhammer, T. R. (2007). Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189, 1417–1425. doi: 10.1128/JB.00904-06
CrossRef Total Text | Google Scholar
Dy, R. L., Richter, C., Salmond, One thousand. P. C., and Fineran, P. C. (2014). Remarkable mechanisms in microbes to resist phage infections. Annu. Rev. Virol. i, 307–331. doi: 10.1146/annurev-virology-031413-085500
CrossRef Full Text | Google Scholar
Faruque, Southward. M., and Mekalanos, J. J. (2003). Pathogenicity islands and phages in Vibrio cholerae evolution. Trends Microbiol. xi, 505–510. doi: x.1016/j.tim.2003.09.003
CrossRef Full Text | Google Scholar
Fineran, P. C., Blower, T. R., Foulds, I. J., Humphreys, D. P., Lilley, K. S., and Salmond, G. P. C. (2009). The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antidote pair. Proc. Natl. Acad. Sci. United statesA. 106, 894–899. doi: 10.1073/pnas.0808832106
CrossRef Full Text | Google Scholar
Garneau, J. E., Dupuis, One thousand. -È, Villion, M., Romero, D. A., Barrangou, R., Boyaval, P., et al. (2010). The CRISPR/Cas bacterial immune organization cleaves bacteriophage and plasmid Deoxyribonucleic acid. Nature 468, 67–71.
Google Scholar
Haurwitz, R. E., Jinek, Yard., Wiedenheft, B., Zhou, K., and Doudna, J. A. (2010). Sequence-and structure-specific RNA processing past a CRISPR endonuclease. Science 329, 1355–1358. doi: x.1126/science.1192272
CrossRef Full Text | Google Scholar
Hood, D. W., Deadman, Chiliad. E., Jennings, M. P., Bisercic, M., Fleischmann, R. D., Venter, J. C., et al. (1996). DNA repeats identify novel virulence genes in Haemophilus influenzae. Proc. Natl. Acad. Sci. The statesA. 93, 11121–11125. doi: 10.1073/pnas.93.xx.11121
CrossRef Full Text | Google Scholar
Huo, Y., Nam, K. H., Ding, F., Lee, H., Wu, Fifty., Xiao, Y., et al. (2014). Structures of CRISPR Cas3 offer mechanistic insights into Cascade-activated Dna unwinding and degradation. Nat. Struct. Mol. Biol. 21, 771–777. doi: 10.1038/nsmb.2875
CrossRef Full Text | Google Scholar
Hyman, P., and Abedon, South. T. (2010). Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70, 217–248. doi: x.1016/S0065-2164(10)70007-one
CrossRef Total Text | Google Scholar
Krüger, D. H., Barcak, 1000. J., and Smith, H. O. (1987). Abolition of DNA recognition site resistance to the restriction endonuclease EcoRII. Biomed. Biochim. Acta 47, K1–K5.
Google Scholar
Labrie, S. J., Samson, J. Due east., and Moineau, S. (2010). Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327. doi: x.1038/nrmicro2315
CrossRef Full Text | Google Scholar
Liu, Chiliad., Deora, R., Doulatov, South. R., Gingery, G., Eiserling, F. A., Preston, A., et al. (2002). Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295, 2091–2094. doi: 10.1126/science.1067467
CrossRef Total Text | Google Scholar
Llosa, M., Gomis-Rüth, F. X., Coll, M., and de la Cruz, F. (2002). Bacterial conjugation: a two-stride machinery for DNA transport. Mol. Microbiol. 45, 1–8. doi: x.1046/j.1365-2958.2002.03014.x
CrossRef Full Text | Google Scholar
Luria, S. E., and Human, M. L. (1952). A nonhereditary, host-induced variation of bacterial viruses. J. Bacteriol. 64, 557.
Google Scholar
Manning, A. J., and Kuehn, G. J. (2011). Contribution of bacterial outer membrane vesicles to innate bacterial defense force. BMC Microbiol. 11:258. doi: 10.1186/1471-2180-11-258
CrossRef Full Text | Google Scholar
Marks, T., and Sharp, R. (2000). Bacteriophages and biotechnology: a review. J. Chem. Technol. Biotechnol. 75, 6–17. doi: x.1002/(SICI)1097-4660(200001)75:1<6::AID-JCTB157>3.0.CO;2-A
CrossRef Full Text | Google Scholar
Marraffini, Fifty. A., and Sontheimer, E. J. (2010). CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat. Rev. Genet. eleven, 181–190. doi: 10.1038/nrg2749
CrossRef Full Text | Google Scholar
Maxwell, K. L. (2016). Phages fight dorsum: inactivation of the CRISPR-Cas bacterial immune system past Anti-CRISPR proteins. PLoS Pathog. 12:e1005282. doi: ten.1371/journal.ppat.1005282
CrossRef Full Text | Google Scholar
McGrath, South., Seegers, J. F. M. L., Fitzgerald, G. F., and van Sinderen, D. (1999). Molecular characterization of a phage-encoded resistance system in Lactococcus lactis. Appl. Environ. Microbiol. 65, 1891–1899.
Google Scholar
Nordström, K., and Forsgren, A. (1974). Effect of protein A on adsorption of bacteriophages to Staphylococcus aureus. J. Virol. fourteen, 198–202.
Google Scholar
Novick, R. P., Christie, Grand. E., and Penadés, J. R. (2010). The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 8, 541–551. doi: 10.1038/nrmicro2393
CrossRef Full Text | Google Scholar
Noyer-Weidner, Chiliad., Diaz, R., and Reiners, L. (1986). Cytosine-specific Dna modification interferes with plasmid establishment in Escherichia coli K12: involvement of rglB. Mol. Gen. Genet. MGG 205, 469–475. doi: 10.1007/BF00338084
CrossRef Total Text | Google Scholar
Pawluk, A., Bondy-Denomy, J., Cheung, V. H. West., Maxwell, K. L., and Davidson, A. R. (2014). A new group of phage anti-CRISPR genes inhibits the type IE CRISPR-Cas system of Pseudomonas aeruginosa. MBio 5:e00896-14. doi: 10.1128/mBio.00896-fourteen
CrossRef Full Text | Google Scholar
Ram, G., Chen, J., Kumar, Thousand., Ross, H. F., Ubeda, C., Damle, P. K., et al. (2012). Staphylococcal pathogenicity island interference with helper phage reproduction is a epitome of molecular parasitism. Proc. Natl. Acad. Sci. U.S.A. 109, 16300–16305. doi: ten.1073/pnas.1204615109
CrossRef Total Text | Google Scholar
Ram, M., Chen, J., Ross, H. F., and Novick, R. P. (2014). Precisely modulated pathogenicity island interference with belatedly phage cistron transcription. Proc. Natl. Acad. Sci. The statesA. 111, 14536–14541. doi: 10.1073/pnas.1406749111
CrossRef Full Text | Google Scholar
Revel, H. R. (1983). DNA modification: glucosylation. Bacteriophage iv, 156–165.
Google Scholar
Roberts, R. J., Belfort, M., Bestor, T., Bhagwat, A. S., Bickle, T. A., Bitinaite, J., et al. (2003). A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 31, 1805–1812. doi: 10.1093/nar/gkg274
CrossRef Full Text | Google Scholar
Ruzin, A., Lindsay, J., and Novick, R. P. (2001). Molecular genetics of SaPI1–a mobile pathogenicity isle in Staphylococcus aureus. Mol. Microbiol. 41, 365–377. doi: ten.1046/j.1365-2958.2001.02488.x
CrossRef Total Text | Google Scholar
Samson, J. East., Magadán, A. H., Sabri, Thousand., and Moineau, S. (2013). Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687. doi: 10.1038/nrmicro3096
CrossRef Full Text | Google Scholar
Sandmeier, H., Iida, South., and Arber, Due west. (1992). DNA inversion regions Min of plasmid p15B and Cin of bacteriophage P1: evolution of bacteriophage tail cobweb genes. J. Bacteriol. 174, 3936–3944.
Google Scholar
Sandmeler, H. (1994). Acquisition and rearrangement of sequence motifs in the evolution of bacteriophage tail fibres. Mol. Microbiol. 12, 343–350. doi: 10.1111/j.1365-2958.1994.tb01023.x
CrossRef Full Text | Google Scholar
Scholl, D., Adhya, S., and Merril, C. (2005). Escherichia coli K1's capsule is a barrier to bacteriophage T7. Appl. Environ. Microbiol. 71, 4872–4874. doi: 10.1128/AEM.71.8.4872-4874.2005
CrossRef Total Text | Google Scholar
Seed, One thousand. D., Faruque, Due south. Grand., Mekalanos, J. J., Calderwood, South. B., Qadri, F., and Camilli, A. (2012). Phase variable O antigen biosynthetic genes control expression of the major protective antigen and bacteriophage receptor in Vibrio cholerae O1. PLoS Pathog. 8:e1002917. doi: x.1371/journal.ppat.1002917
CrossRef Full Text | Google Scholar
Seed, 1000. D., Lazinski, D. Due west., Calderwood, S. B., and Camilli, A. (2013). A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494, 489–491. doi: ten.1038/nature11927
CrossRef Full Text | Google Scholar
Shi, Y., Yan, Y., Ji, W., Du, B., Meng, X., Wang, H., et al. (2012). Label and conclusion of holin protein of Streptococcus suis bacteriophage SMP in heterologous host. Virol. J. 9, lxx. doi: 10.1186/1743-422X-9-70
CrossRef Total Text | Google Scholar
Snyder, L. (1995). Phage-exclusion enzymes: a bonanza of biochemical and prison cell biology reagents? Mol. Microbiol. 15, 415–420. doi: 10.1111/j.1365-2958.1995.tb02255.x
CrossRef Full Text | Google Scholar
Stern, A., and Sorek, R. (2011). The phage-host arms race: shaping the evolution of microbes. Bioessays 33, 43–51. doi: 10.1002/bies.201000071
CrossRef Full Text | Google Scholar
Stewart, F. J., Panne, D., Bickle, T. A., and Raleigh, E. A. (2000). Methyl-specific Deoxyribonucleic acid binding by McrBC, a modification-dependent restriction enzyme. J. Mol. Biol. 298, 611–622. doi: x.1006/jmbi.2000.3697
CrossRef Full Text | Google Scholar
Sturino, J. M., and Klaenhammer, T. R. (2004). Bacteriophage defence force systems and strategies for lactic acid bacteria. Adv. Appl. Microbiol. 56, 332–378.
Google Scholar
Thomas, C. M., and Nielsen, K. Grand. (2005). Mechanisms of, and barriers to, horizontal cistron transfer between bacteria. Nat. Rev. Microbiol. 3, 711–721. doi: 10.1038/nrmicro1234
CrossRef Total Text | Google Scholar
Tock, Grand. R., and Dryden, D. T. F. (2005). The biological science of restriction and anti-brake. Curr. Opin. Microbiol. 8, 466–472. doi: x.1016/j.mib.2005.06.003
CrossRef Full Text | Google Scholar
van Duijn, E., Barbu, I. G., Barendregt, A., Jore, M. Thou., Wiedenheft, B., Lundgren, K., et al. (2012). Native tandem and ion mobility mass spectrometry highlight structural and modular similarities in amassed-regularly-interspaced shot-palindromic-repeats (CRISPR)-associated protein complexes from Escherichia coli and Pseudomonas aeruginosa. Mol. Cell. Proteomics eleven, 1430–1441. doi: ten.1074/mcp.M112.020263
CrossRef Total Text | Google Scholar
Weiss, A. A., Hewlett, E. L., Myers, Thou. A., and Falkow, S. (1983). Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42, 33–41.
Google Scholar
Westra, E. R., van Erp, P. B. G., Künne, T., Wong, S. P., Staals, R. H. J., Seegers, C. L. C., et al. (2012). CRISPR immunity relies on the sequent bounden and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol. Prison cell 46, 595–605. doi: ten.1016/j.molcel.2012.03.018
CrossRef Total Text | Google Scholar
Wiedenheft, B., van Duijn, E., Bultema, J. B., Waghmare, S. P., Zhou, Thou., Barendregt, A., et al. (2011). RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc. Natl. Acad. Sci. U.s.A. 108, 10092–10097. doi: 10.1073/pnas.1102716108
CrossRef Total Text | Google Scholar
Wilson, G. G., and Murray, North. Due east. (1991). Restriction and modification systems. Annu. Rev. Genet. 25, 585–627. doi: 10.1146/annurev.ge.25.120191.003101
CrossRef Full Text | Google Scholar
Wommack, K. E., and Colwell, R. R. (2000). Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64, 69–114. doi: x.1128/MMBR.64.one.69-114.2000
CrossRef Full Text | Google Scholar
Zaleski, P., Wojciechowski, M., and Piekarowicz, A. (2005). The function of Dam methylation in phase variation of Haemophilus influenzae genes involved in defence against phage infection. Microbiology 151, 3361–3369. doi: 10.1099/mic.0.28184-0
CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fmicb.2016.01292/full
0 Response to "Bacteria Defense Against Phage Nature Review Microbiology Crispr Rm Abi"
Post a Comment